Description
O2k-Chamber: 16 mm inner diameter, Duran glass polished, with standard operation volume (V) of 2 mL (2 cm3). The O2k-sV-Module has a small chamber volume of 0.5 mL.
Reference: O2k-Chamber
MitoPedia O2k and high-resolution respirometry:
O2k-Open Support
O2k-Chamber: paradigm shift in HRR
- The glass chamber and PVDF stirrer of the O2k.
- O2k: Paradigm shift and features of high-resolution respirometry. MiPNet09.01. Including sole source information. Bioblast Link
- Small experimental chamber volumes of 20-50 µL generate problems rather than providing resolution with small amounts of tissue samples, low numbers of cultured cells or few isolated mitochondria. The surface to volume ratio increases with decreasing chamber volume, thus various boundary effects entail larger errors at smaller volume, in particular oxygen diffusion. While the rate of oxygen depletion per unit amount of sample increases linearly with decreasing chamber volume, side effects may increase to a larger degree. Then, sensitivity is lost with decreasing chamber size. We recommend a minimum chamber volume of 0.5 mL of the O2k-sV-Module or 2.0 mL for the operational volume of the standard O2k-Chamber.
Paradigm shift from mimimum to optimum chamber volume
- 1. Minimization of chamber volume represents a past paradigm, aming at high rates of oxygen consumption per volume. The advantage appears to be obvious, whereas the drawbacks are frequently overlooked (see below).
- 2. Advancements of electronics, data acquisition and analysis, polarographic oxygen sensor specifications and chamber design made possible a superior approach, allowing for respirometric measurements at high dilution, as reviewed by Gnaiger E (2001). In specifically designed mitochondrial respiration media, respiration is stable at high dilution, multiple substrate/inhibitor titrations are possible without oxygen depletion, and a low-oxygen regime may be chosen to prevent elevation of oxidative stress at air-level oxygen saturation. In contrast, micro-chambers are characterized by a high surface-to-volume ratio which hinders optimum stirring, increases unfavourable surface effects and oxygen-backdiffusion, and poses problems with accurate titrations and dilution effects of the sample. These potential - and mostly hidden - artefacts are avoided in high-resolution respirometry, using glass chambers, titanium stoppers, and avoiding teflon-coated stirrers or perspex (yielding high back-diffusion of oxygen).
- Assume you have 0.1 mg mitochondrial protein for a respirometric assay. Approach (1) would lead you to search for a 100 µL volume respirometer, to maintain a classical 1 mg/mL protein concentration. In contrast, high-resolution respirometry allows for dilution of mitochondria to 0.02 mg/mL protein. Dilution of 0.1 mg mitochondrial protein in a 2 mL chamber yields an optimum concentration for multiple substrate/inhibitor titrations and kinetic measurements. The high-resolution approach of the Oroboros O2k offers the unique advantages of a versatile and ready-to-use system for studies in mitochondrial physiology and pathology.
Cleaning the glass chamber
- There are different kinds of contamination that can accumulate in the measuring chamber of the oxygraph and cause problems. All of them have to be treated in different ways:
- Biological contamination: The ideal counter agent is 70% Ethanol with 30% water (NOT 100% Ethanol). If this does not help, the biological contamination may be embedded in e.g. protein contamination and the glass chamber should be disassembled and cleaned as described below:
- Protein contamination and other macroscopic deposits: After long term use, a whitish deposit can be formed on the glass walls of the O2k-Chamber. Additionally, small glass splinters (difficult to see) may become embedded in such deposit. A sign of this is a jumping stirring bar or a stirring bar getting stuck. In this case the glass chamber should be removed from the O2k and treated with concentrated hydrochloric acid (HCL) for at least an overnight period. Use a stirring plate and the O2k-Stirring bar to simultaneously clean the stirrer bar.
- Contamination by hydrophobic inhibitors: The ideal counter agent for this case is pure ethanol.
- Carry-over of inhibitors or uncouplers: Inhibitors bind to the mitochondria, thus, it is recommended to wash the O2k-Chambers with cells, tissue or isolated mitochondria stored at -20°C. If Living cells (ce) are available, these are preferred due to their higher binding capacity. Fill the O2k-Chambers to the rim with the suspension and leave in the O2k-Chambers with inserted stoppers for at least 30 minutes. Follow this with a cleaning of the O2k-Chambers according to the DLPs provided with the DatLab software. See here.
- Inhibitors may be introduced accidentally, one example being 70% ethanol used in hospital settings containing antiseptics. Such inhibitors may accumulate e.g. in plastic parts and inhibit subsequent experiments. See the Discussion page.
- There are different kinds of contamination that can accumulate in the measuring chamber of the oxygraph and cause problems. All of them have to be treated in different ways:
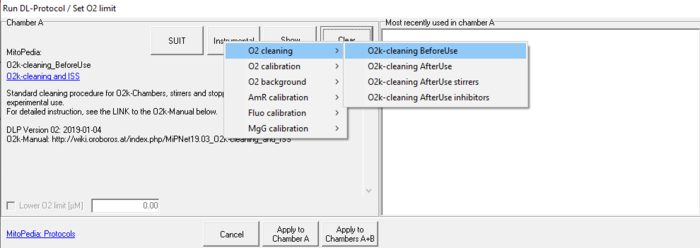
Cleaning DLP
DL-Protocols (DLP) should be used for cleaning the O2k-Chambers, stirrers and stoppers before and after each experimental use. A full list of Instrumental DL-Protocols for cleaning is displayed in the 'Protocols' menu ('Run DL-Protocol / Set O2 limit' window). See Browse Instrumental DL-Protocols for cleaning
O2k-Chamber assembly - possible problems
- A properly assembled O2k-Chamber can remain in the O2k for extended periods of time.
- It is recommended to disassemble, clean and reassemble the O2k-Chamber at least every 1 - 2 years, to avoid the chamber from getting stuck, to clean the glass properly, and to detect any possible damages.
- After assembly, medium is leaking out of the chamber
- Siphon off medium and disassemble the chamber.
- Check for apparent damages of the glass chamber, the POS seal tip and O-rings on chamber holders and stoppers. If any damage is visible, replace the part.
- Reassemble the chamber and proceed with an O2 sensor test.
- Oxygen leak of the chamber (strongly negative intercept in the instrumental background test
- Unstable O2 signal
- Unexpected high or low O2 signal
- The possible contribution of chamber assembly is checked by removing the OroboPOS from the chamber and observing, if the signal changes to the expected values. - » Futher details.
- If the O2k signal responds as expected, reassemble the O2k-chamber and proceed with an O2 sensor test.
Leaky chamber due to damaged OroboPOS-Holder
- Please see: O2k-OroboPOS-Holder MitoPedia
Chamber disassembly
- After siphoning off any medium from the chamber, remove the OroboPOS-Connector and O2k-Chamber Holder from the O2k.
- Insert your finger (with gloves on) into the chamber and then gently pull the glass chamber up.
- Problem: Glass chamber stuck in the O2k, and the O2k-Chamber cannot be removed. It remains stuck even after washing with water.
- This may be caused by spilled medium acting as an adhesive between glass chamber and copper block. Wash with plenty of warm water and try to remove the chamber while the O2k is switched on and heated up to 37 °C. Then GENTLY screw in the sensor holder (not the sensor connector) to push the glass chamber upwards (as you do during chamber_assembly.
- If this does not work, increase the temperature to 40 °C (or slightly higher) for washing and continue with removing attempts.
Video support for Chamber service
- Instructions on how to insert the chamber and how to volume calibrate can be found here: Chamber service
MitoPedia O2k and high-resolution respirometry: O2k hardware