Pesta 2011 Am J Physiol Regul Integr Comp Physiol
| Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E (2011) Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301:R1078–87. |
Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E (2011) Am J Physiol Regul Integr Comp Physiol
Abstract: Endurance and strength training are established as distinct exercise modalities, increasing either mitochondrial density or myofibrillar units. Recent research, however, suggests that mitochondrial biogenesis is stimulated by both training modalities. To test the training-"specificity" hypothesis, mitochondrial respiration was studied in permeabilized muscle fibers from 25 sedentary adults after endurance (ET) or strength training (ST) in normoxia or hypoxia (FiO2=21% or 13.5%). Biopsies were taken from the m. vastus lateralis and cycle-ergometric incremental VO2max exercise tests were performed under normoxia, before and after the 10-week training program. The main finding was a significant increase (P<0.05) of tissue-specific fatty acid oxidation capacity, after endurance and strength training under normoxia (2.6- and 2.4-fold for ETN and STN; N=8 and 3) and hypoxia (2.0-fold for ETH and STH; N=7 and 7), and higher coupling control of oxidative phosphorylation. The enhanced lipid OXPHOS capacity was mainly (87%) due to qualitative mitochondrial changes increasing the relative capacity for fatty acid oxidation (P<0.01). Mitochondrial tissue-density contributed to a smaller extent (13%), reflected by the gain in tissue-specific respiratory capacity with a physiological substrate cocktail (glutamate, malate, succinate, octanoylcarnitine). No significant increase was observed in mtDNA content. Physiological OXPHOS capacity increased significantly in ETN (P<0.01), with the same trend in ETH and STH (P<0.1). The limitation of flux by the phosphorylation system was diminished after training. Importantly, key mitochondrial adaptations were similar after endurance and strength training, regardless of normoxic or hypoxic exercise. The transition from a sedentary to an active life style induced muscular changes of mitochondrial quality representative of mitochondrial health. • Keywords: Mitochondrial respiration, Endurance training, Strength training, Human skeletal muscle, Permeabilized fibers, OXPHOS capacity, Coupling control, Fatty acid oxidation
• O2k-Network Lab: AT Innsbruck Gnaiger E, AT Innsbruck Burtscher M, AT Innsbruck MitoCom
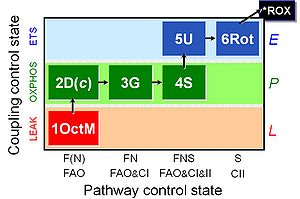
SUIT protocol
Correction
- Tab. 2: For N=25, some average values need to be corrected:
- Js: ETFL (OctM) correct value: 7.1 +- 1.6 (instead of 6.0 +- 2.0)
- Js: CI+IIP (GMSOct) correct value: 99.4 +- 20.9 (instead of 86.3 +- 17.9)
- Tab. 2: For N=25, some average values need to be corrected:
- Fig. 4 C and D: The correct caption of the Y-axis is: ET capacity (CI+IIE).
- Methods (p. R1081): FCCP was titrated at 0.25-µM steps (not 0.025 µM-steps).
MitoEAGLE VO2max/BME data base
- Human vastus lateralis
- 25 males
- 26 years
- Sedentary
- H = 1.82 m
- M = 84.3 kg
- BME = 1.21
- BMI = 25.6 kg·m-2
- VO2max/BM = 40.8 mL·min-1·kg-1
- Permeabilized muscle fibres; 37 °C; GMSP; mw
- JO2,P(NS) = 76.8 µmol·s-1·kg-1 wet muscle mass
- Human vastus lateralis
- 25 males
- 26 years
- Sedentary/10 weeks trained
- H = 1.82 m
- M = 81.0 kg
- BME = 1.16
- BMI = 24.6 kg·m-2
- VO2max/BM = 44.0 mL·min-1·kg-1
- Permeabilized muscle fibres; 37 °C; GMSP; mw
- JO2,P(NS) = 86.3 µmol·s-1·kg-1 wet muscle mass
O2k-Publications
References: BME and VO2max
- » VO2max
| Reference | |
|---|---|
| Bakkman 2007 ActaPhysiol | Bakkman L, Sahlin K, Holmberg HC, Tonkonogi M (2007) Quantitative and qualitative adaptation of human skeletal muscle mitochondria to hypoxic compared with normoxic training at the same relative work rate. Acta Physiol (Oxford) 190:243–51. |
| Boushel 2007 Diabetologia | Boushel RC, Gnaiger E, Schjerling P, Skovbro M, Kraunsoee R, Dela F (2007) Patients with Type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50:790-6. |
| Chambers 2020 J Appl Physiol (1985) | Chambers TL, Burnett TR, Raue U, Lee GA, Finch WH, Graham BM, Trappe TA, Trappe S (2020) Skeletal muscle size, function, and adiposity with lifelong aerobic exercise. J Appl Physiol (1985) 128:368–78. |
| Daussin 2008 Am J Physiol Regul Integr Comp Physiol | Daussin FN, Zoll J, Dufour SP, Ponsot E, Lonsdorfer-Wolf E, Doutreleau S, Mettauer B, Piquard F, Geny B, Richard R (2008) Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: relationship to aerobic performance improvements in sedentary subjects. Am J Physiol Regul Integr Comp Physiol 295:R264-72. |
| Garnier 2005 FASEB J | Garnier A, Fortin D, Zoll J, N'Guessan B, Mettauer B, Lampert E, Veksler V, Ventura-Clapier R (2005) Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J 19:43-52. |
| Gnaiger 2015 Scand J Med Sci Sports | Gnaiger E, Boushel R, Søndergaard H, Munch-Andersen T, Damsgaard R, Hagen C, Díez-Sánchez C, Ara I, Wright-Paradis C, Schrauwen P, Hesselink M, Calbet JAL, Christiansen M, Helge JW, Saltin B (2015) Mitochondrial coupling and capacity of oxidative phosphorylation in skeletal muscle of Inuit and caucasians in the arctic winter. https://doi.org/10.1111/sms.12612 |
| Gnaiger 2019 MiP2019 | OXPHOS capacity in human muscle tissue and body mass excess – the MitoEAGLE mission towards an integrative database (Version 6; 2020-01-12). |
| Loe 2013 PLOS ONE | Loe H, Rognmo Ø, Saltin B, Wisløff U (2013) Aerobic capacity reference data in 3816 healthy men and women 20-90 years. PLOS ONE 8:e64319. |
| Mettauer 2001 J Am Coll Cardiol | Mettauer B, Zoll J, Sanchez H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R (2001) Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J Am Coll Cardiol 38:947-54. |
| Mogensen 2006 J Physiol | Mogensen M, Bagger M, Pedersen PK, Fernström M, Sahlin K (2006) Cycling efficiency in humans is related to low UCP3 content and to type I fibres but not to mitochondrial efficiency. J Physiol 571:669-81. |
| N'Guessan 2004 Mol Cell Biochem | N'Guessan B, Zoll J, Ribera F, Ponsot E, Lampert E, Ventura-Clapier R, Veksler V, Mettauer B (2004) Evaluation of quantitative and qualitative aspects of mitochondrial function in human skeletal and cardiac muscles. Mol Cell Biochem 256-257:267-80. |
| Pesta 2011 Am J Physiol Regul Integr Comp Physiol | Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E (2011) Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301:R1078–87. |
| Ponsot 2006 J Appl Physiol (1985) | Ponsot E, Dufour SP, Zoll J, Doutrelau S, N'Guessan B, Geny B, Hoppeler H, Lampert E, Mettauer B, Ventura-Clapier R, Richard R (2006) Exercise training in normobaric hypoxia in endurance runners. II. Improvement of mitochondrial properties in skeletal muscle. J Appl Physiol (1985) 100:1249-57. |
| Pribis 2010 Nutrients | Pribis P, Burtnack CA, McKenzie SO, Thayer J (2010) Trends in body fat, body mass index and physical fitness among male and female college students. Nutrients 2:1075-85. |
| Raboel 2009 Diabetes Obes Metab | Raboel R, Hojberg PM, Almdal T, Boushel RC, Haugaard SB, Madsbad S, Dela F (2009) Improved glycaemic control decreases inner mitochondrial membrane leak in type 2 diabetes. Diabetes Obes Metab 11:355-60. |
| Rasmussen 2001 Am J Physiol Endocrinol Metab | Rasmussen UF, Rasmussen HN, Krustrup P, Quistorff B, Saltin B, Bangsbo J (2001) Aerobic metabolism of human quadriceps muscle: in vivo data parallel measurements on isolated mitochondria. Am J Physiol Endocrinol Metab 280:E301-7. |
| Rasmussen 2003 Eur J Physiol | Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN (2003) Human skeletal muscle mitochondrial metabolism in youth and senescence: no signs of functional changes in ATP formation and mitochondrial oxidative capacity. Pflugers Arch – Eur J Physiol 446:270-78. |
| Zoll 2002 J Physiol | Zoll J, Sanchez H, N'Guessan B, Ribera F, Lampert E, Bigard X, Surrurier B, Fortin D, Geny B, Veksler V, Ventura-Clapier R, Mettauer B (2002) Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 543:191-200. |
Labels: MiParea: Respiration, mt-Biogenesis;mt-density, Exercise physiology;nutrition;life style
Pathology: Obesity
Stress:Hypoxia
Organism: Human
Tissue;cell: Skeletal muscle
Preparation: Permeabilized tissue
Regulation: Coupling efficiency;uncoupling, Fatty acid Coupling state: LEAK, OXPHOS, ET Pathway: F, N, S, NS HRR: Oxygraph-2k
Ergometry, VO2max, 1OctM;2D;3G;4S;5U;6Rot-, SUIT-017, BMI, BME, MitoEAGLE BME