ET capacity: Difference between revisions
No edit summary |
No edit summary |
||
| (124 intermediate revisions by 9 users not shown) | |||
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=''E'' | |abbr=''E'' | ||
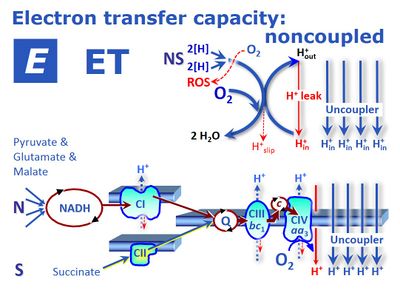
|description= | |description=[[File:E.jpg]] '''T capacity''' is the respiratory electron-transfer-pathway capacity ''E'' of mitochondria measured as oxygen consumption in the noncoupled state at optimum [[uncoupler]] concentration. This optimum concentration is obtained by stepwise titration of an established protonophore to induce maximum oxygen flux as the determinant of ET capacity. The experimentally induced noncoupled state at optimum uncoupler concentration is thus distinguished from (''1'') a wide range of uncoupled states at any experimental uncoupler concentration, (''2'') physiological uncoupled states controlled by intrinsic uncoupling (e.g. UCP1 in brown fat), and (''3'') pathological dyscoupled states indicative of mitochondrial injuries or toxic effects of pharmacological or environmental substances. ET capacity in mitochondrial preparations requires the addition of defined fuel substrates to establish an ET-pathway competent state. | ||
| | » [[#Why ET capacity, why not State 3u.3F | '''MiPNet article''']] | ||
| | |info=[[BEC 2020.1]], [[Gnaiger 2020 BEC MitoPathways]], [[Gnaiger 2009 Int J Biochem Cell Biol]] | ||
}} | |||
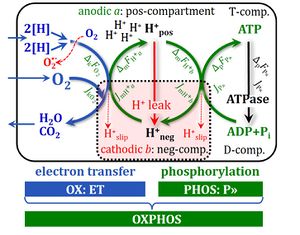
[[File:ETS-NS.jpg|400px|thumb|Noncoupled respiration with a shortcircuit of the proton cycle across the inner mt-membrane at optimum uncoupler (protonophore) concentration stimulating maximum oxygen flux. 2[H] indicates the reduced hydrogen equivalents of CHO substrates and electron transfer to oxygen. H<sup>+</sup><sub>out</sub> are protons pumped out of the matrix phase. Proton leaks dissipate the energy of translocated protons. ET capacity is not limited by the capacity of the phosphorylation system (uncontrolled state). Measurement of ET capacity is possible by uncoupler titrations in living cells and in mt-preparations supported by an ET-pathway competent state, exemplified as the NS-electron transfer pathway state (CI<small>&</small>II-linked substrate supply). Modified after [[Gnaiger 2020 BEC MitoPathways]]).]] | |||
__TOC__ | |||
= Why ET capacity, why not State 3u? = | |||
{{Publication | |||
|title=Gnaiger E (2017) Why ET capacity, why not State 3u? Mitochondr Physiol Network (2014-07-06) last update 2020-11-10. | |||
|info= | |||
|authors=Oroboros | |||
|year=2017 | |||
|journal=MiPNet | |||
|abstract=[[File:E.jpg]] Measurement of '''ET capacity''' in the noncoupled state at optimum uncoupler concentration does not represent a general substitute for determination of [[OXPHOS-capacity]] (compare State 3). If the ratio of OXPHOS/ET capacity (OXPHOS-control ratio or ''P/E'' ratio) is less than one, noncoupled respiration overestimates the apparent reserve capacity for oxidative phosphorylation with respect to ROUTINE-respiration of living cells. The conditions for measurement and expression of respiration ''E'' vary (oxygen flux, ''J''<sub>O<sub>2</sub>''E''</sub>, or oxygen flow, ''I''<sub>O<sub>2</sub>''E''</sub>). If these conditions are defined and remain consistent within a given context, then the simple symbol ''E'' is used to substitute the more explicit expression for respiratory capacity. In the ET state, the [[mt-membrane potential]] is almost fully collapsed and provides a reference state for [[flux control ratio]]s. | |||
|mipnetlab=AT Innsbruck Gnaiger E | |||
}} | |||
== The important difference between rates ''P'' and ''E'' == | |||
:::: The abbreviation '''[[State 3u]]''' is used frequently in bioenergetics, to indicate the noncoupled state of maximum respiration with the rate ''E'',<ref>Gnaiger E. Electron transfer pathway versus electron transport chain. Mitochondr Physiol Network. »[[Electron transfer pathway]]«</ref> without sufficient emphasis on the fundamental difference between ''P'' (OXPHOS-capacity; coupled, with an uncoupled component) and ''E'' (ET capacity, noncoupled) (Gnaiger 2009, 2020). | |||
::::* '''''P''=''E''''': The specific case of equal OXPHOS- and ET capacity (''P/E''=1) yields the important information that the capacity of the [[phosphorylation system]] matches or is in potential excess of the ET capacity, such that OXPHOS-capacity is not limited by the phosphorylation system in a specific electron transfer pathway state. This varies with species and tissues, and changes as a result of pathologies due to defects in the phosphorylation system. An example for ''P/E''=1 is mouse skeletal muscle mitochondria (Aragones et al 2008). | |||
::::* '''''P''<''E''''': When OXPHOS capacity is less than ET capacity, the phosphorylation system limits OXPHOS-capacity, and there is an apparent ''E-P'' excess capacity. For example, this is the case in healthy human skeletal muscle mitochondria (Pesta et al 2011) and even more pronounced in human cardiac mitochondria (Lemieux et al 2011). | |||
::::* '''''P''>''E''''': If ET capacity is less than OXPHOS capacity in living cells, or in mitochondrial preparations with defined substrate(s), then you have encountered an experimental artefact, and the apparent ET capacity is too low. Artificially low ET capacity may be obtained due to over-titration of uncoupler, which inhibits the ET pathway. Inhibitors of ATP synthase (oligomycin) may suppress ET capacity in living cells, particularly in stressed cells ([[Doerrier 2018 Methods Mol Biol]]). | |||
== Consequences for evaluation of coupling == | |||
:::: In some textbooks on Bioenergetics, the respiratory acceptor control ratio RCR is defined as either the State 3/State 4 ratio or the State 3u/State 4 ratio. This reflects lack of conceptual distinction between State 3 (compare ''P'') and 3u (''E''), and clarification is best achieved by avoiding ambiguous terminology. RCR as defined originally is the 'acceptor control ratio' or 'adenylate control ratio', State 3/State 4 (compare LEAK-control ratio, ''L/E''; [[biochemical coupling efficiency]]). The UCR may be defined as the ''E''/''L'' or ''E''/''R'' coupling-control ratio. ET capacity but not OXPHOS capacity provides a generally valid reference for an index of uncoupling. | |||
== References == | |||
<references/> | |||
:::# Aragonés J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, K Harten S, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P (2008) Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40:170-80. [[Aragones 2008 Nat Genet |»Bioblast link«]] | |||
:::# Gnaiger E. Biochemical coupling efficiency: from 0 to <1. Mitochondr Physiol Network. - »[[Biochemical coupling efficiency]]« | |||
:::# Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45. - [[Gnaiger 2009 Int J Biochem Cell Biol |»Bioblast link«]] | |||
:::# Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002 | |||
:::# Gnaiger Erich et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1 | |||
:::# Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962) Studies on the '''Electron transfer pathway''' XLII. Reconstitution of the Electron transfer pathway. J Biol Chem 237:2661-9. - [[Hatefi 1962 J Biol Chem-XLII |»Bioblast link«]] | |||
:::# International Union of Biochemistry (1991) Nomenclature of '''electron-transfer proteins.''' Biochim Biophys Acta 1060. [http://www.chem.qmul.ac.uk/iubmb/etp/ »Open Access«] | |||
:::# International Union of Biochemistry and Molecular Biology. Recommendations for terminology and databases for biochemical thermodynamics - The IUPAC Green Book. [http://www.chem.qmul.ac.uk/iubmb/thermod2/ »Open Access«] | |||
:::# Lemieux H, Semsroth S, Antretter H, Höfer D, Gnaiger E (2011) Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol 43:1729–38. - [[Lemieux 2011 Int J Biochem Cell Biol |»Bioblast link«]] | |||
:::# Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E (2011) Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301:R1078–87. [[Pesta 2011 Am J Physiol Regul Integr Comp Physiol |»Bioblast link«]] | |||
::::* [[Bioblast_alert_2014#Bioblast_alert_2014.2804.29:_2014-07-07|Bioblast alert 2014(04)]] | |||
::::* [[Bioblast_alert_2013#Bioblast_alert_2013.2802.29:_2013-08-08|Bioblast alert 2013(02)]]: '''[[CCCP]]''' versus FCCP for high-resultion respirometry ([[HRR]]). | |||
== Keywords == | |||
{{Template:Keywords: Coupling control}} | |||
{{Keywords: Uncoupling}} | |||
[[Image:MiPMap Publication.jpg|120px|link=http://www.bioblast.at/index.php/MiPMap|Publications in the MiPMap]] | |||
[[File:Expand.png|right|45px |Click to expand or collaps]] | |||
<div class="toccolours mw-collapsible mw-collapsed"> | |||
::: '''» List of publications: ET pathway ''' | |||
<div class="mw-collapsible-content"> | |||
'''''Sort in ascending/descending order by a click on one of the small symbols in squares below'''''. | |||
Default sorting: chronological. Empty fields appear first in ascending order. | |||
{{#ask:[[Category:Publications]] [[Coupling states::ET]] | |||
|?Was published in year=Year | |||
|?Has title=Reference | |||
|?Coupling states=Coupling | |||
|?Pathways=Pathway | |||
|?Preparation=Preparation | |||
|?Mammal and model=Organism | |||
|?Tissue and cell=Tissue;cell | |||
|format=broadtable | |||
|limit=5000 | |||
|offset=0 | |||
|sort=Was published in year | |||
|order=descending | |||
}} | |||
[[Image:MiPMap Publication.jpg|left|120px|link=http://www.bioblast.at/index.php/MiPMap|Publications in the MiPMap]] | |||
: '''Abstracts: ET pathway ''' | |||
'''''Sort in ascending/descending order by a click on one of the small symbols in squares below'''''. | |||
{{#ask:[[Category:Abstracts]] [[Coupling states::ET]] | |||
|?Was submitted in year=Year | |||
|?Has title=Reference | |||
|?Coupling states=Coupling | |||
|?Pathways=Pathway | |||
|?Preparation=Preparation | |||
|?Mammal and model=Organism | |||
|?Tissue and cell=Tissue;cell | |||
|format=broadtable | |||
|limit=5000 | |||
|offset=0 | |||
|sort=Was submitted in year | |||
|order=descending | |||
}} | |||
</div> | |||
</div> | |||
{{#ask:[[Additional label::ET capacity]] | |||
| mainlabel=Bioblast link | |||
|?Has title=Reference | |||
|?Was published in year=Year | |||
|format=broadtable | |||
|limit=5000 | |||
|offset=0 | |||
|sort=Has title | |||
|order=ascending | |||
}} | |||
<br /> | |||
{{MitoPedia concepts | |||
|mitopedia concept=MiP concept, Respiratory state, Recommended | |||
}} | }} | ||
{{MitoPedia methods | {{MitoPedia methods | ||
|mitopedia method=Respirometry | |mitopedia method=Respirometry | ||
}} | }} | ||
{{MitoPedia topics | {{MitoPedia topics | ||
|mitopedia topic= | |mitopedia topic=Uncoupler, EAGLE | ||
| | }} | ||
{{Labeling | |||
|couplingstates=ET | |||
|instruments=Theory | |||
}} | }} | ||
Latest revision as of 20:30, 19 March 2022
Description
[[Description::![]() T capacity is the respiratory electron-transfer-pathway capacity E of mitochondria measured as oxygen consumption in the noncoupled state at optimum uncoupler concentration. This optimum concentration is obtained by stepwise titration of an established protonophore to induce maximum oxygen flux as the determinant of ET capacity. The experimentally induced noncoupled state at optimum uncoupler concentration is thus distinguished from (1) a wide range of uncoupled states at any experimental uncoupler concentration, (2) physiological uncoupled states controlled by intrinsic uncoupling (e.g. UCP1 in brown fat), and (3) pathological dyscoupled states indicative of mitochondrial injuries or toxic effects of pharmacological or environmental substances. ET capacity in mitochondrial preparations requires the addition of defined fuel substrates to establish an ET-pathway competent state.
» MiPNet article]]
T capacity is the respiratory electron-transfer-pathway capacity E of mitochondria measured as oxygen consumption in the noncoupled state at optimum uncoupler concentration. This optimum concentration is obtained by stepwise titration of an established protonophore to induce maximum oxygen flux as the determinant of ET capacity. The experimentally induced noncoupled state at optimum uncoupler concentration is thus distinguished from (1) a wide range of uncoupled states at any experimental uncoupler concentration, (2) physiological uncoupled states controlled by intrinsic uncoupling (e.g. UCP1 in brown fat), and (3) pathological dyscoupled states indicative of mitochondrial injuries or toxic effects of pharmacological or environmental substances. ET capacity in mitochondrial preparations requires the addition of defined fuel substrates to establish an ET-pathway competent state.
» MiPNet article]]
Abbreviation: Has abbr::''E''
Reference: [[Info::BEC 2020.1, Gnaiger 2020 BEC MitoPathways, Gnaiger 2009 Int J Biochem Cell Biol]]
Why ET capacity, why not State 3u?
| Has title::Gnaiger E (2017) Why ET capacity, why not State 3u? Mitochondr Physiol Network (2014-07-06) last update 2020-11-10. |
Was written by::Oroboros (Was published in year::2017) Was published in journal::MiPNet
Abstract: [[has abstract::![]() Measurement of ET capacity in the noncoupled state at optimum uncoupler concentration does not represent a general substitute for determination of OXPHOS-capacity (compare State 3). If the ratio of OXPHOS/ET capacity (OXPHOS-control ratio or P/E ratio) is less than one, noncoupled respiration overestimates the apparent reserve capacity for oxidative phosphorylation with respect to ROUTINE-respiration of living cells. The conditions for measurement and expression of respiration E vary (oxygen flux, JO2E, or oxygen flow, IO2E). If these conditions are defined and remain consistent within a given context, then the simple symbol E is used to substitute the more explicit expression for respiratory capacity. In the ET state, the mt-membrane potential is almost fully collapsed and provides a reference state for flux control ratios.]]
Measurement of ET capacity in the noncoupled state at optimum uncoupler concentration does not represent a general substitute for determination of OXPHOS-capacity (compare State 3). If the ratio of OXPHOS/ET capacity (OXPHOS-control ratio or P/E ratio) is less than one, noncoupled respiration overestimates the apparent reserve capacity for oxidative phosphorylation with respect to ROUTINE-respiration of living cells. The conditions for measurement and expression of respiration E vary (oxygen flux, JO2E, or oxygen flow, IO2E). If these conditions are defined and remain consistent within a given context, then the simple symbol E is used to substitute the more explicit expression for respiratory capacity. In the ET state, the mt-membrane potential is almost fully collapsed and provides a reference state for flux control ratios.]]
• O2k-Network Lab: Was published by MiPNetLab::AT Innsbruck Gnaiger E
The important difference between rates P and E
- The abbreviation State 3u is used frequently in bioenergetics, to indicate the noncoupled state of maximum respiration with the rate E,[1] without sufficient emphasis on the fundamental difference between P (OXPHOS-capacity; coupled, with an uncoupled component) and E (ET capacity, noncoupled) (Gnaiger 2009, 2020).
- P=E: The specific case of equal OXPHOS- and ET capacity (P/E=1) yields the important information that the capacity of the phosphorylation system matches or is in potential excess of the ET capacity, such that OXPHOS-capacity is not limited by the phosphorylation system in a specific electron transfer pathway state. This varies with species and tissues, and changes as a result of pathologies due to defects in the phosphorylation system. An example for P/E=1 is mouse skeletal muscle mitochondria (Aragones et al 2008).
- P<E: When OXPHOS capacity is less than ET capacity, the phosphorylation system limits OXPHOS-capacity, and there is an apparent E-P excess capacity. For example, this is the case in healthy human skeletal muscle mitochondria (Pesta et al 2011) and even more pronounced in human cardiac mitochondria (Lemieux et al 2011).
- P>E: If ET capacity is less than OXPHOS capacity in living cells, or in mitochondrial preparations with defined substrate(s), then you have encountered an experimental artefact, and the apparent ET capacity is too low. Artificially low ET capacity may be obtained due to over-titration of uncoupler, which inhibits the ET pathway. Inhibitors of ATP synthase (oligomycin) may suppress ET capacity in living cells, particularly in stressed cells (Doerrier 2018 Methods Mol Biol).
Consequences for evaluation of coupling
- In some textbooks on Bioenergetics, the respiratory acceptor control ratio RCR is defined as either the State 3/State 4 ratio or the State 3u/State 4 ratio. This reflects lack of conceptual distinction between State 3 (compare P) and 3u (E), and clarification is best achieved by avoiding ambiguous terminology. RCR as defined originally is the 'acceptor control ratio' or 'adenylate control ratio', State 3/State 4 (compare LEAK-control ratio, L/E; biochemical coupling efficiency). The UCR may be defined as the E/L or E/R coupling-control ratio. ET capacity but not OXPHOS capacity provides a generally valid reference for an index of uncoupling.
References
- ↑ Gnaiger E. Electron transfer pathway versus electron transport chain. Mitochondr Physiol Network. »Electron transfer pathway«
- Aragonés J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, K Harten S, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P (2008) Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40:170-80. »Bioblast link«
- Gnaiger E. Biochemical coupling efficiency: from 0 to <1. Mitochondr Physiol Network. - »Biochemical coupling efficiency«
- Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45. - »Bioblast link«
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- Gnaiger Erich et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1
- Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962) Studies on the Electron transfer pathway XLII. Reconstitution of the Electron transfer pathway. J Biol Chem 237:2661-9. - »Bioblast link«
- International Union of Biochemistry (1991) Nomenclature of electron-transfer proteins. Biochim Biophys Acta 1060. »Open Access«
- International Union of Biochemistry and Molecular Biology. Recommendations for terminology and databases for biochemical thermodynamics - The IUPAC Green Book. »Open Access«
- Lemieux H, Semsroth S, Antretter H, Höfer D, Gnaiger E (2011) Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol 43:1729–38. - »Bioblast link«
- Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E (2011) Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301:R1078–87. »Bioblast link«
- Bioblast alert 2014(04)
- Bioblast alert 2013(02): CCCP versus FCCP for high-resultion respirometry (HRR).
Keywords
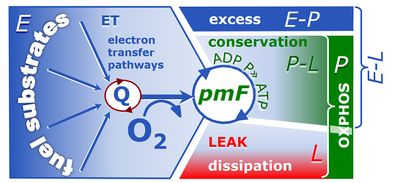
4-compartmental OXPHOS model. (1) ET capacity E of the noncoupled electron transfer system ETS. OXPHOS capacity P is partitioned into (2) the dissipative LEAK component L, and (3) ADP-stimulated P-L net OXPHOS capacity. (4) If P-L is kinetically limited by a low capacity of the phosphorylation system to utilize the protonmotive force pmF, then the apparent E-P excess capacity is available to drive coupled processes other than phosphorylation P» (ADP to ATP) without competing with P».
- Bioblast links: Coupling control - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
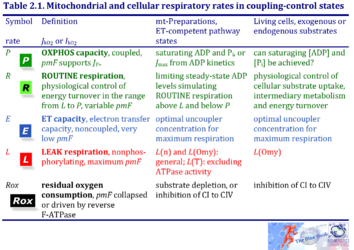
1. Mitochondrial and cellular respiratory rates in coupling-control states
| Respiratory rate | Defining relations | Icon | |
|---|---|---|---|
| OXPHOS capacity | P = P´-Rox | mt-preparations | |
| ROUTINE respiration | R = R´-Rox | living cells | |
| ET capacity | E = E´-Rox | » Level flow | |
| » Noncoupled respiration - Uncoupler | |||
| LEAK respiration | L = L´-Rox | » Static head | |
| » LEAK state with ATP | |||
| » LEAK state with oligomycin | |||
| » LEAK state without adenylates | |||
| Residual oxygen consumption Rox | L = L´-Rox |
2. Flux control ratios related to coupling in mt-preparations and living cells
| FCR | Definition | Icon | |
|---|---|---|---|
| L/P coupling-control ratio | L/P | » Respiratory acceptor control ratio, RCR = P/L | |
| L/R coupling-control ratio | L/R | ||
| L/E coupling-control ratio | L/E | » Uncoupling-control ratio, UCR = E/L (ambiguous) | |
| P/E control ratio | P/E | ||
| R/E control ratio | R/E | » Uncoupling-control ratio, UCR = E/L | |
| net P/E control ratio | (P-L)/E | ||
| net R/E control ratio | (R-L)/E |
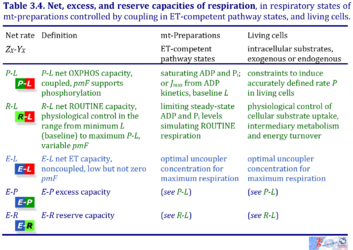
3. Net, excess, and reserve capacities of respiration
| Respiratory net rate | Definition | Icon |
|---|---|---|
| P-L net OXPHOS capacity | P-L | |
| R-L net ROUTINE capacity | R-L | |
| E-L net ET capacity | E-L | |
| E-P excess capacity | E-P | |
| E-R reserve capacity | E-R |
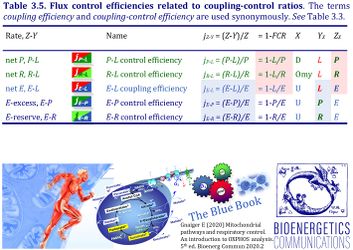
4. Flux control efficiencies related to coupling-control ratios
| Coupling-control efficiency | Definition | Icon | Canonical term | ||
|---|---|---|---|---|---|
| P-L control efficiency | jP-L | = (P-L)/P | = 1-L/P | P-L OXPHOS-flux control efficiency | |
| R-L control efficiency | jR-L | = (R-L)/R | = 1-L/R | R-L ROUTINE-flux control efficiency | |
| E-L coupling efficiency | jE-L | = (E-L)/E | = 1-L/E | E-L ET-coupling efficiency » Biochemical coupling efficiency | |
| E-P control efficiency | jE-P | = (E-P)/E | = 1-P/E | E-P ET-excess flux control efficiency | |
| E-R control efficiency | jE-R | = (E-R)/E | = 1-R/E | E-R ET-reserve flux control efficiency |
5. General
- » Basal respiration
- » Cell ergometry
- » Dyscoupled respiration
- » Dyscoupling
- » Electron leak
- » Electron-transfer-pathway state
- » Hyphenation
- » Oxidative phosphorylation
- » Oxygen flow
- » Oxygen flux
- » Permeabilized cells
- » Phosphorylation system
- » Proton leak
- » Proton slip
- » Respiratory state
- » Uncoupling
- Bioblast links: Uncoupling - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Specific
- » Artefacts by single dose uncoupling
- » ATP synthase
- » CCCP
- » Coupling-control protocol
- » DNP
- » Dyscoupled respiration
- » FCCP
- » Is respiration uncoupled - noncoupled - dyscoupled?
- » Noncoupled respiration: Discussion
- » Uncoupler
- » Uncoupled respiration - see » Noncoupled respiration
- » Uncoupling proteins
- » Uncoupling protein 1
- » Uncoupler titrations - Optimum uncoupler concentration
- Specific
- Respiratory states and control ratios
- » Biochemical coupling efficiency
- » Coupling-control state
- » Electron-transfer-pathway state
- » Electron-transfer pathway
 ET capacity
ET capacity- » E-L coupling efficiency
- » Flux control efficiency
- » Flux control ratio
- » LEAK-control ratio
- » LEAK respiration
- » Noncoupled respiration
- » OXPHOS
- » OXPHOS capacity; » State 3
- » OXPHOS-control ratio, P/E ratio
- » Respiratory acceptor control ratio
- » ROUTINE-control ratio
- » ROUTINE respiration
- » ROUTINE state
- » State 3u
- » State 4
- » Uncoupling-control ratio UCR
- Respiratory states and control ratios
- Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- General (alphabetical order)
- Other keyword lists
- » List of publications: ET pathway
Sort in ascending/descending order by a click on one of the small symbols in squares below. Default sorting: chronological. Empty fields appear first in ascending order.
{{#ask: Coupling states::ET |?Was published in year=Year |?Has title=Reference |?Coupling states=Coupling |?Pathways=Pathway |?Preparation=Preparation |?Mammal and model=Organism |?Tissue and cell=Tissue;cell |format=broadtable |limit=5000 |offset=0 |sort=Was published in year |order=descending }}
- Abstracts: ET pathway
Sort in ascending/descending order by a click on one of the small symbols in squares below.
{{#ask: Coupling states::ET |?Was submitted in year=Year |?Has title=Reference |?Coupling states=Coupling |?Pathways=Pathway |?Preparation=Preparation |?Mammal and model=Organism |?Tissue and cell=Tissue;cell |format=broadtable |limit=5000 |offset=0 |sort=Was submitted in year |order=descending }}
{{#ask:Additional label::ET capacity | mainlabel=Bioblast link |?Has title=Reference |?Was published in year=Year |format=broadtable |limit=5000 |offset=0 |sort=Has title |order=ascending }}
MitoPedia concepts:
MiP concept,
Respiratory state,
Recommended
MitoPedia methods:
Respirometry
MitoPedia topics:
Uncoupler,
MitoPedia topic::EAGLE
Labels:
Coupling state: Coupling states::ET
HRR: Instrument and method::Theory